Biography
Complexes containing metal ions in oxygen, nitrogen and/or sulfur environments serve as natural and industrial catalysts. One goal of my research includes the formation and study of metal carboxylates as copolymerization catalysts. The copolymerization of carbon dioxide and epoxides require a catalyst to form aliphatic polycarbonates. This reaction is very important since it represents an environmentally benign synthetic route to biodegradable thermoplastics which does not require bisphenol A. Bisphenol A is an endocrine disruptor and has possible negative effects on human. Bisphenol A has been found in the human blood supply and is believed to have leached from polycarbonate bottles.
Copolymerization catalysts that have shown promise in the literature contain the metals zinc, cobalt, aluminum, and chromium with organic ligands such as alkoxides, carboxylates, porphyrins, salens, and beta-diminates. The following benzoic acid derived ligands 1(R=H) and 2(R=H) are being synthesized and characterized in my laboratory. Once synthesized these ligands will be reacted with zinc to form complexes where the zinc is bonded to carboxylate and alkoxides. These complexes will be used to catalyze the copolymerization of cyclohexene oxide (1,2- epoxycyclohexane) and carbon dioxide.
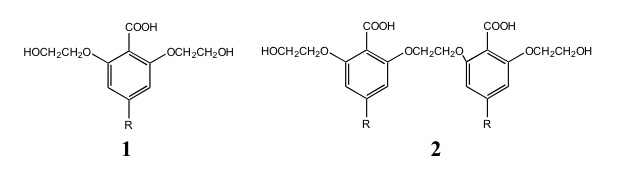
It has been reported in the literature that when two different catalysts containing single zinc ions were added together in the copolymerization reaction, that the activity doubled even though the total zinc concentration had not changed from previous reactions. It was proposed that the two zinc complexes formed a dimer under the reaction conditions and that this dizinc dimer caused the increased activity. Ligand 2 has the ability to bond with one or two zinc ions. The comparison of copolymerization activity of monozinc and dizinc complexes will be examined in my laboratory. This work would help to establish in the literature whether dimetal complexes or monometal complexes are the better catalysts for polymerization of epoxides and carbon dioxide.
Students have determined the best methyl esterification method for the starting material, 2,6 dihydroxybenzoic acid. Literature yields for this compound range from 25-55%. We used a new method and produced methyl 2,6-dihydroxybenozoate in a yield of 70%. We synthesized and characterized 1(R=H) and , 2(R=H) . We have also attempted to make the zinc complex using 2(R=H), but results were inconclusive because of solubility problems. Currently we are maximizing the yield of 2(R=H) and preparing to synthesize the zinc complex. The zinc complex will then be used as a catalyst for the copolymerization between carbon dioxide and cyclohexene oxide to form the polycarbonate.